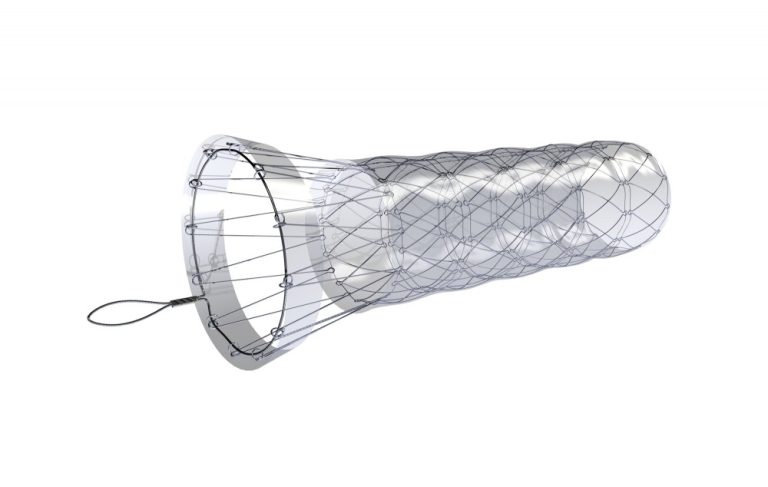
Boubella-E is a metallic, self-expandable stent made of stainless steel. The stent is composed of individual connected segments with a funnel-shaped, partially uncovered anti-migration flare. The stent body is already completely covered with polyethylene film. Optionally with or without antireflux valve.
Boubella-E is designed for relieving malignant or benign esophageal strictures if standard operation is contraindicated. The variant with an antireflux valve is intended primarily for the middle and distal part of the esophagus in cases where the stent passes through the cardia. It can be used for treatment of esophagorespiratory fistulas as well.
Boubella-E is made of stainless steel wire. The stent is composed of separate segments/ cells, thanks to which it retains its excellent expansion force and does not elongate during compression.
The stent design ensures effectiveness even in very rigid stenoses and, thanks to the massive anti-migration segment, reduces the risk of dislocation and migration.
Full coverage of the stent with polyethylene film prevents the ingrowth of tumor tissue and allows the esophagorespiratory fistulas to be covered and sealed.
The larger diameter of the proximal flare ensures tightness even in the partially dilated esophagus above the stenosis. The uncovered part of the stent flare provides stent fixation in esophageal tissue and reduces dislocation/ migration.
The option with an anti-reflux valve works to prevent gastroesophageal reflux and possible aspiration.
The retrieval loop at the proximal stent end is made of a special alloy and is characterized by high resistance and strength.
The ferromagnetic material of the stent is characterized by excellent radiopacity/ visibility under fluoroscopic control.
MRI compatibility - the stent is made of ferromagnetic material, thus the product is classified as MR Unsafe.
The stent is supplied sterile and already compressed in a 750 mm long 28F / 18F (9.4mm / 6.0mm) delivery system. Stent insertion must be performed under fluoroscopic control
When the stent is released, the "splittable olive" at the end of the delivery system is released and split at the same time, thus avoiding the risk of the delivery system not being able to be withdrawn if the stent does not immediately expand to a sufficient diameter in a rigid stenosis.
For the implantation, we recommend using a very rigid (ultra stiff) guidewire 0.035” (0.89 mm) with a length of 220 cm.
The proximal anti-migration segment must be placed above the stenosis / outside the stricture in healthy tissue.