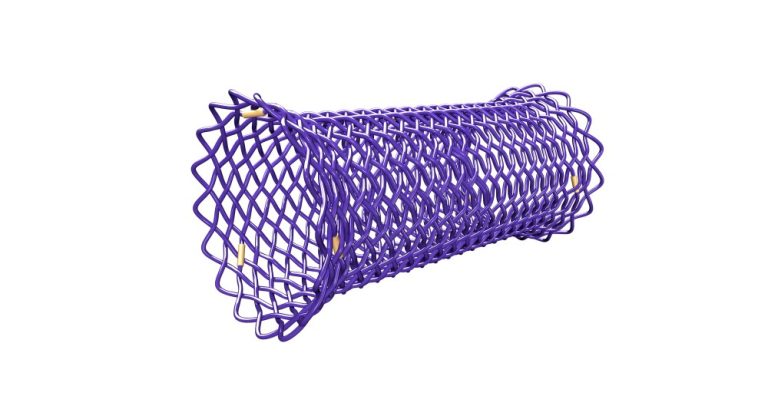
The BD Stent is a worldwide unique self-expandable esophageal biodegradable stent that degrades in the human body usually 3 to 4 months after the implantation. Therefore, it is an ideal solution for temporary use in benign indications, where it is not necessary to extract it from the body like other non-degradable stents (including possible extraction from the stomach due to stent migration). In specific cases, custom-made production is possible.
BD Stent is intended for patients aged 18 and older and is indicated for management of refractory benign esophageal stenoses:
The BD Stent degrades in the human body usually in 3 to 4 months. This reduces the number of interventions and hospital visits.
The stent is made of polydioxanone, an absorbable material (polymer) used in medicine as a surgical suture for more than 30 years.
Stent integrity and radial force are maintained for 6 to 8 weeks after implantation. Subsequently, the radial force gradually decreases until the degradation of the material after 3 to 4 months.
The pH value affects the stent degradation process. At higher pH, stent degradation is slower, at lower pH, stent degradation is faster.
Low migration is achieved by uncovered stent design.
Polydioxanone is not visible under X-ray, so the stent is equipped with gold markers - 3 pieces at both ends and 1 piece at the midpoint of the stent.
MRI compatibility - "MR Conditional", compatible with 1.5 Tesla and 3 Tesla static magnetic field.
IMPLANTATION
The stent is supplied sterile and is packaged separately in addition to the delivery system and compressor (ie, a means for easier compression of the stent into the delivery system). Just prior to implantation, the stent must be manually compressed into the delivery system. We recommend using a 0.035 ”(0.89 mm) / 220 cm ultra stiff guidewire for the implantation.
Daisy Walter, Maarten W. Van Den Berg, Meike M. Hirdes, Frank P. Vleggaar, Alessandro Repici, Pierre H. Deprez, Laurence Lovat, Bartolomé L. Viedma, Bas L. Weusten, Raf Bisschops, Renan Haidry, Elisa Ferrara, Keith J. Sanborn, Erin E. O’Leary, Jeanin E. Van Hooft, Peter D. Siersema
Endoscopy. 2018 Mar. Georg Thieme Verlag KG Stuttgart. New York, ISSN 0013-726X
D. Esposito, F. Calabrese, L. Fanti, E. Viale, P.A. Testoni Abstracts of the 24th National Congress of Digestive Diseases / Digestive and Liver Disease 50/S2 (2018) e63–e238
Nogales Óscar, Clemente Ana, Caballero-Marcos Aránzazu, García-Lledó Javier, Pérez-Carazo Leticia, Merino Beatriz, López-Ibáñez María, Pérez Valderas María Dolores, Bañares Rafael, González-Asanza Cecilia
M. M. C. Hirdes, P. D. Siersema, P. G. A. van Boeckel, F. P. Vleggaar Endoscopy. 2012 Jul;44(7):649-54.